![]()
Education and Training
Did You Know?...
True or False? Once I have IRB approval, I can access University of Maryland Medical System (UMMS) patient information for my research.
Answer: False. IRB approval does not grant access to patient information. IRB approval refers to the determination by an Institutional Review Board (IRB) that a research study has been reviewed and may be conducted at an institution. Access to patient information must be granted by UMMS. Per the Health Insurance Portability and Accountability Act (HIPAA,) UMMS is required to track all instances where patient information was accessed for research purposes. To access UMMS patient information, a request must be submitted to the UMMS Research Informatics Core (RIC) through the ICTR Resource Request system. The IRB determination letter will be required before the RIC team will grant access via chart review or release data files for the purposes of research, including screening for eligibility. The RIC team may determine that there are other requirements, such as a Data Use Agreement (DUA).
More resources and information to be added soon!
UMB ICTR/CTSA Training and Education
Other Training & Learning Opportunities
-

NIH Tips on Grant Writing and Developing a Strong Application
GRANTS & Funding
NIH Central Resource for Grants and Funding Information
Search UMB ICTR
Contact
UMB ICTR
The University of Maryland, Baltimore collaborates with Johns Hopkins University for the prestigious National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA).



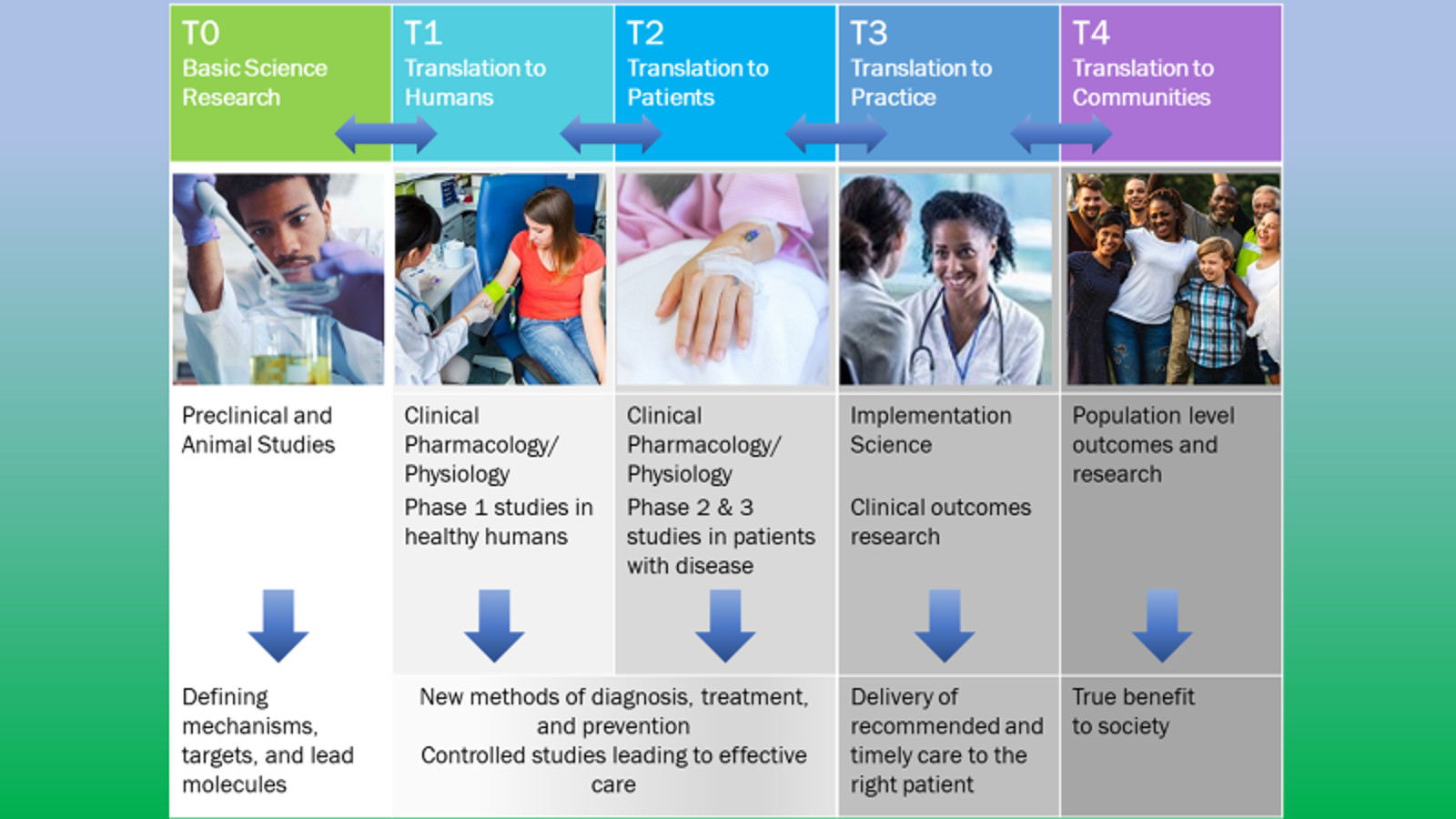

.jpg)




